SZTE Nanotechnological Research Group
Principal investigator: Dr. Rita Ambrus habil PhD
Projects
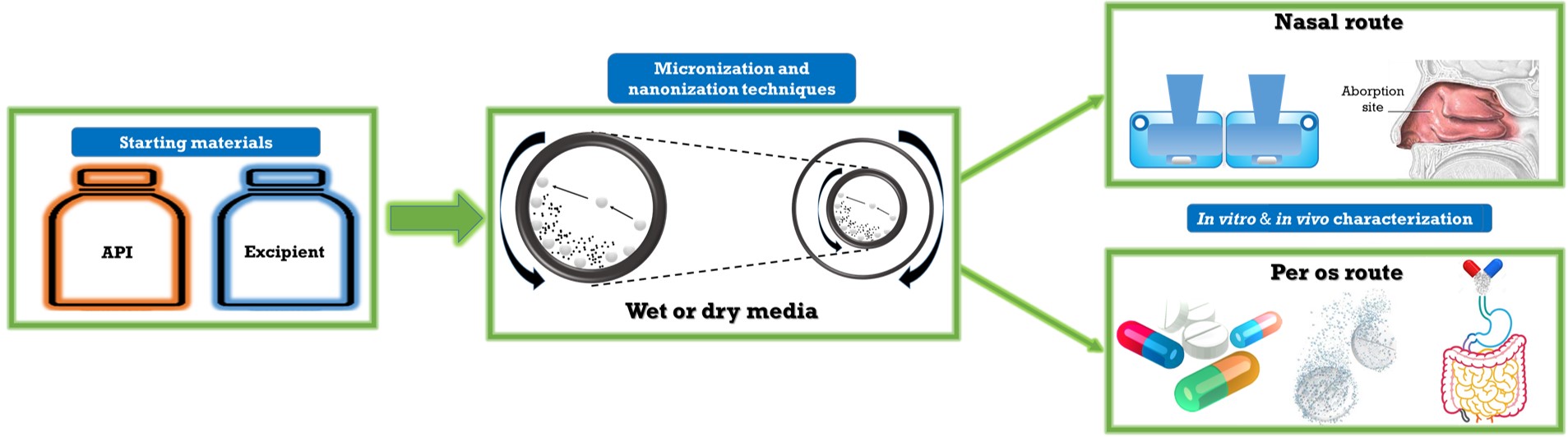
Milling is a commonly applied technique to produce micro- or nanosized drug crystals. The main effect of the process is the comminution of particles, which results in changes in physicochemical properties of the ground material. The combined wet milling process used also in this research work is a fusion of two basic milling techniques, in both cases the milling is performed in presence of grinding media (beads). One of the aims of this work was to study the wet milling process, where the planetary ball mill was combined with pearl milling technology and optimize the process parameters and predict the robustness of the process and design the amount of the additive (PVA) in order to produce NSAID containing nanosuspension without any pre-treating procedure. In other way, we produced dry powder formulation containing micro- or nanosized drugs (which have effect in central nervous system) with additives, as a further applicable intranasal formulations.
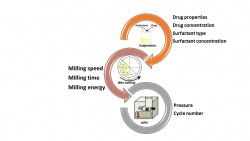
Nanosization is one of the most applied technologies used to improve solubility and dissolution of poorly water-soluble drugs. Combination technology (CT) has also been developed as a new method to prepare nanocrystals. CT combines wet bead milling and high pressure homogenization (HPH) can maximize particle reduction for better dissolution and solubility. Besides, overcome the limitations of the single methods. Therefore, nanocrystals might have higher dissolution and solubility hence bioavailability compared to unprocessed microparticles. This project aims to investigate the feasibility of nanocrystals preparation of different active pharmaceutical ingredients. Two non-steroidal anti-inflammatory drugs -meloxicam and niflumic acid- are subjected to the present investigation.
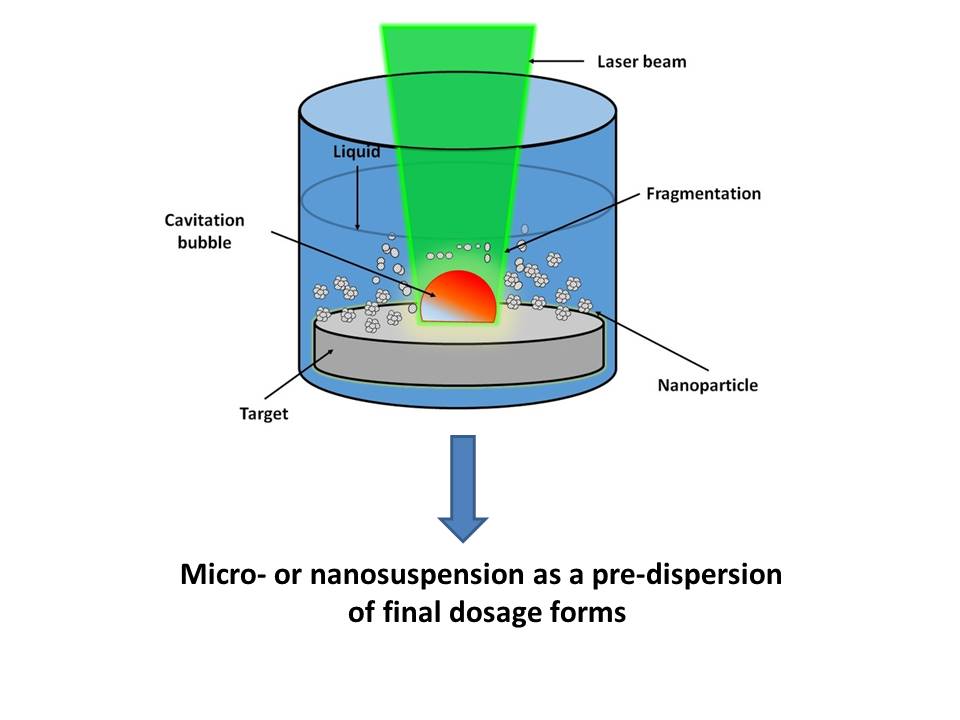
During our work a poorly water-soluble meloxicam was milled first time by pulsed laser ablation in liquid, which is a novel possibility to prepare a micro- or nanosized dispersion as an intermediate product/predispersion. The effect of the applied wavelengths (λ = 532 nm), fluence value (energy density 4,5 J/cm2) and polymer type (PVP, PVA, Poloxamer) on the habit, structure, solubility and in vitro properties of the drug were investigated.
During our work a poorly water-soluble meloxicam was milled first time by pulsed laser ablation in liquid, which is a novel possibility to prepare a micro- or nanosized dispersion as an intermediate product/predispersion. The effect of the applied wavelengths (λ = 532 nm), fluence value (energy density 4,5 J/cm2) and polymer type (PVP, PVA, Poloxamer) on the habit, structure, solubility and in vitro properties of the drug were investigated.
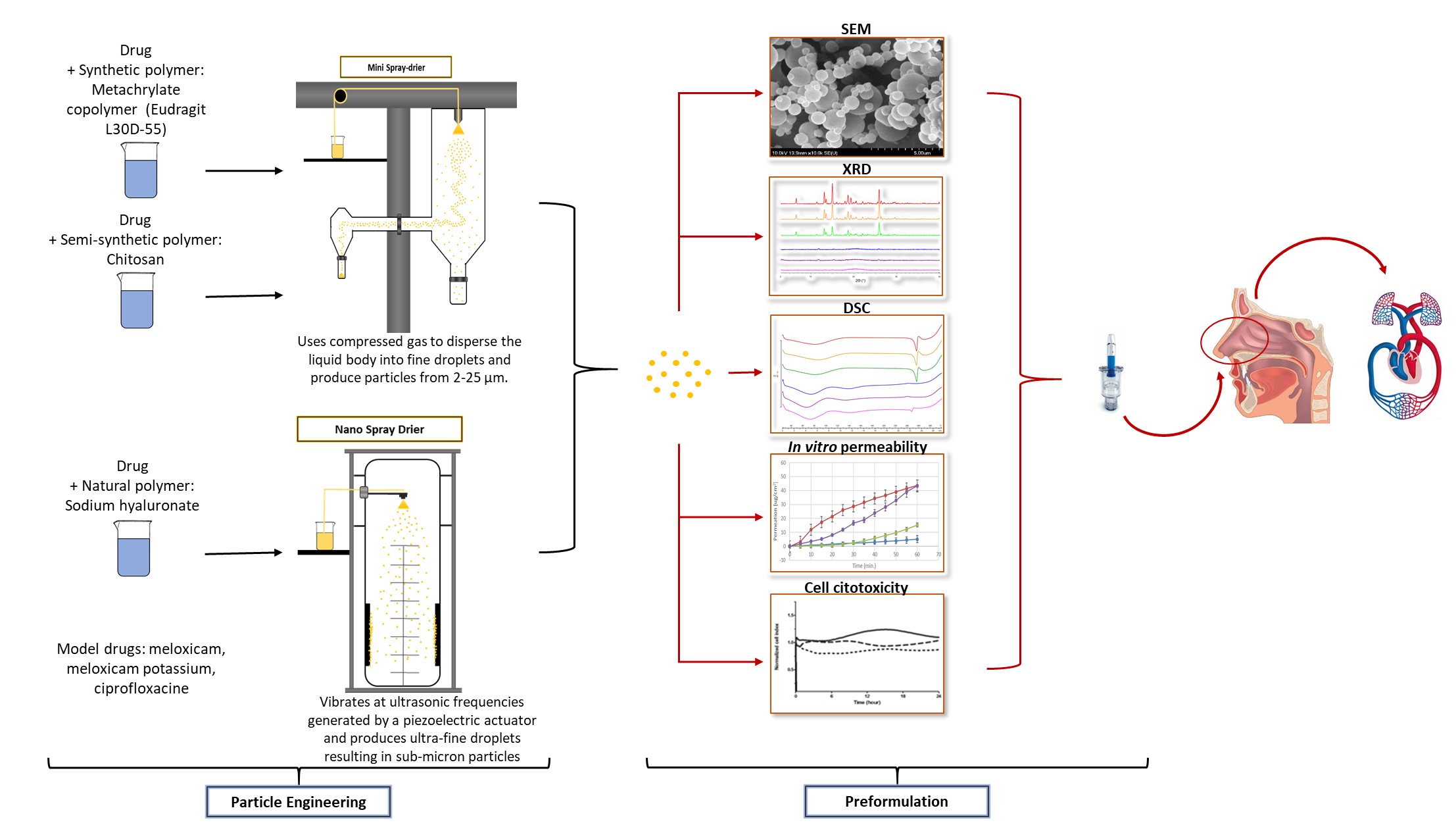
Encapsulation of poorly water-soluble drug with low dissolution rate and inadequate permeability in a polymer carrier by spray drying can increase its bioavailability. Furthermore, incorporation of drug provides protection against harmful external effects and may ensure controlled drug release. Synthetic, semi-synthetic and natural polymers can be applied for encapsulation of drugs. Micro-or nanpoarticles can also be produced by spray-drying.
The aim of our work is to formulate micro- and nanospheres intended for intranasal application using spray drying methods. Büchi Mini B-290 srpay dryer was applied for preparation of microparticles. Büchi Nano B-90 HP will be used for formulation of nanoparticles. We would like to set the parameters of drying procedures using different polymers (methacrylate copolymer, chitosan and sodium hyaluronate) as encapsulation additives and various acitve ingredients. The physico-chemical properties and the citotxicity of micro- and nanospheres will be investigated. In vitro dissolution and diffusion of products will be determined and compared.
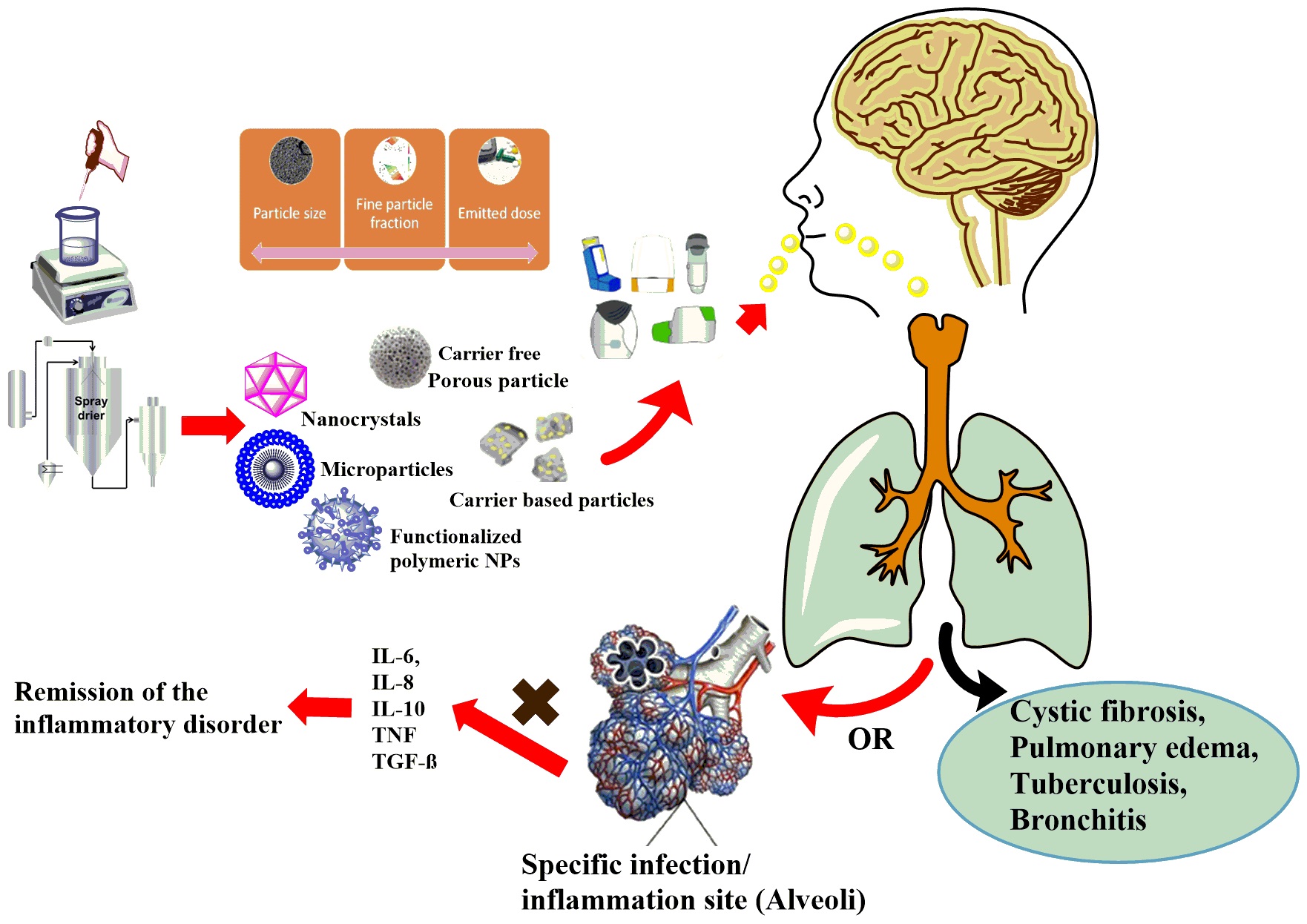
Pulmonary drug delivery (PDD) has several benefits over oral medications. Thus, it is becoming an alternative choice to treat local and systemic diseases. PDDs can be divided into three main categories (pressured metered-dose inhalers, nebulizers, dry powder inhalers /DPIs/).
Most of the DPIs available on the market are made with carrier-based formulation, which involves applying the active ingredient particles to the surface of a large carrier particle by forming an interactive physical mixture. In the case of carrier-free DPI systems, the application of large carriers can be avoided with the use of special excipients and technologies (e.g. co-spray drying). These systems yield around 50–60% Fine Particle Fraction results due to the apparent high cohesive properties between the active ingredient's particles. These formulations involve the usage of different amino acids (e.g. L-leucine) and polymers (e.g. polyvinyl alcohol or polyvinylpyrrolidone). In addition to the various excipients used, formulation of nanosuspension and than nano spray-drying could be a new option for the preparation of carrier-free DPIs. Durring our work NSAID and antibiotic agents are used for the treatment of CF and COPD.
Other aim of this research filed is to fabricate the targeted drug delivery nano vehicle for the active targeting of the inflamed pulmonary tissues. Different polymers and their derivatives are to be assessed to establish their potential in the targeted drug delivery. The rationale of the active targeted delivery is the sustained release of drug to the affected cells while minimizing the drug associated toxicity to the healthy epithelium. This targeted nanotherapeutics might be able to target the disease at the molecular level with regression and remission. This may lead to the inhibition of cascade of cellular events involved in the stimulation and aggravation of inflammation.
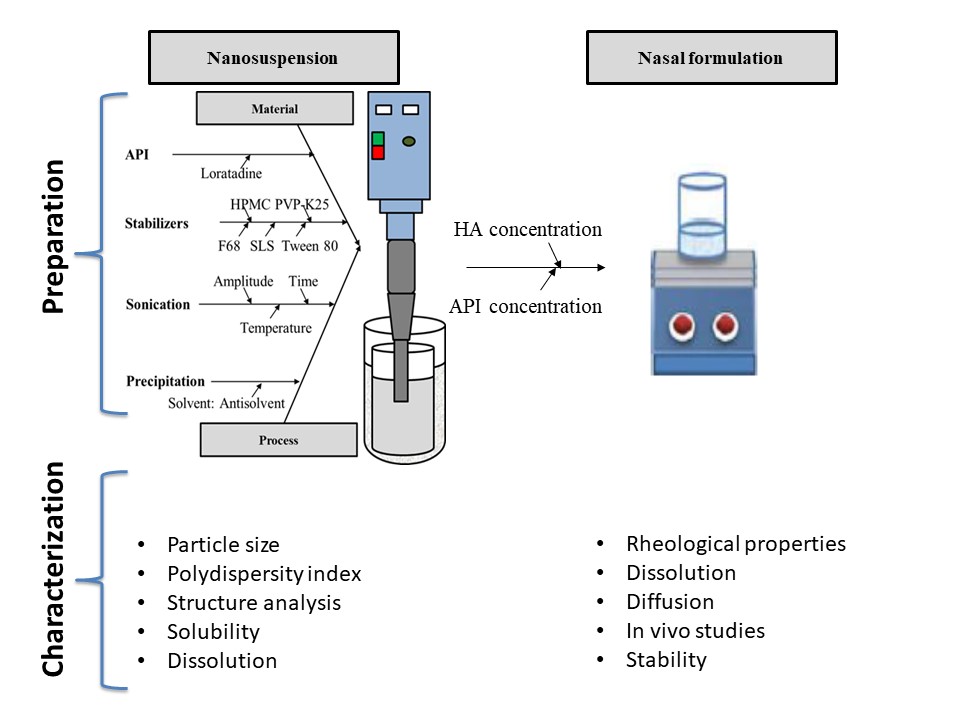
This project mainly designed to fabricate nanosuspension into nasal dosage form as an alternative administration route of loratadine. The production process compromised the formulation of Loratadine nanosuspension as a pre-dispersion by antisolvent ultrasonication method, preparation of the mucoadhesive agent solution and combination of the nanosuspension to the mucoadhesive solution.
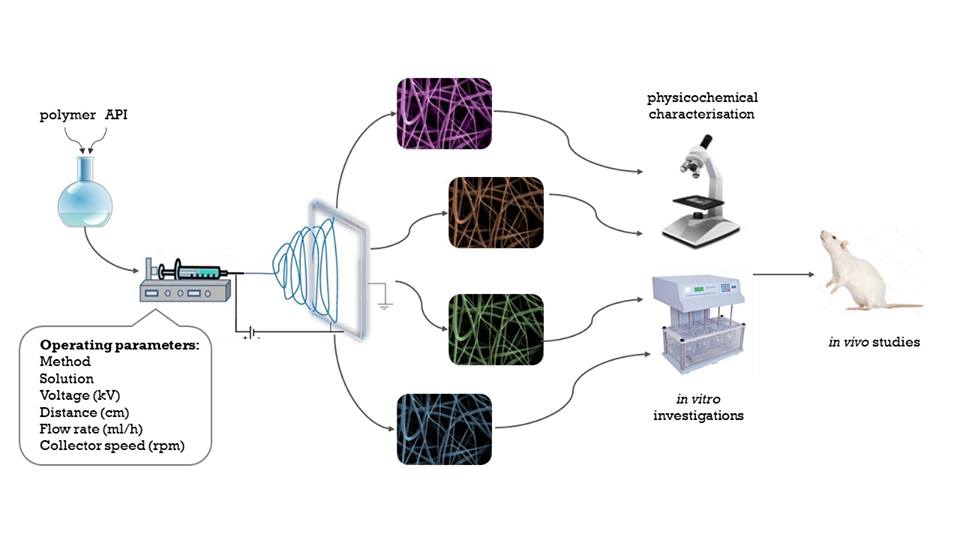
Nowadays one of the main aims of pharmaceutical technology is increasing the water solubility of BCS II and BCS IV drugs. To achieve that various nanonization techniques have been developed. One of this methods is electrospinning. During electrospinning procedure polymeric fibers with diameters from nano- to micrometers can be made by using electric force. The large surface area, high degree of porosity and amorphous active pharmaceutical ingredient (API) prove the increased water solubility and dissolution rate of the API. The structure and morphology of the electrospun fibers can be varied by using different operating parameters such as method, polymer-API solution, applied voltage, tip-to-collector distance, flow rate and the collector speed. The tested nanofibers were loaded with ciprofloxacin, niflumic acid and loratadine as a model drugs.
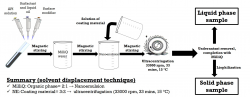
Nanocapsules (NCs) a type of polymeric nanoparticles has become one of the most researched nanosturctured systems in the past years due to their advantageous properties and versatile appilication. Those are able to enhance the bioavailabiliy of hydrophobic drugs by impoving their permeability, solubility and NCs can protect them from the physiological environment (e.g. enzymatic degradation, pH change). They can be used via many drug administration ways e.g. oral, nasal and ocular formulations are exisiting in liquid and solid forms.
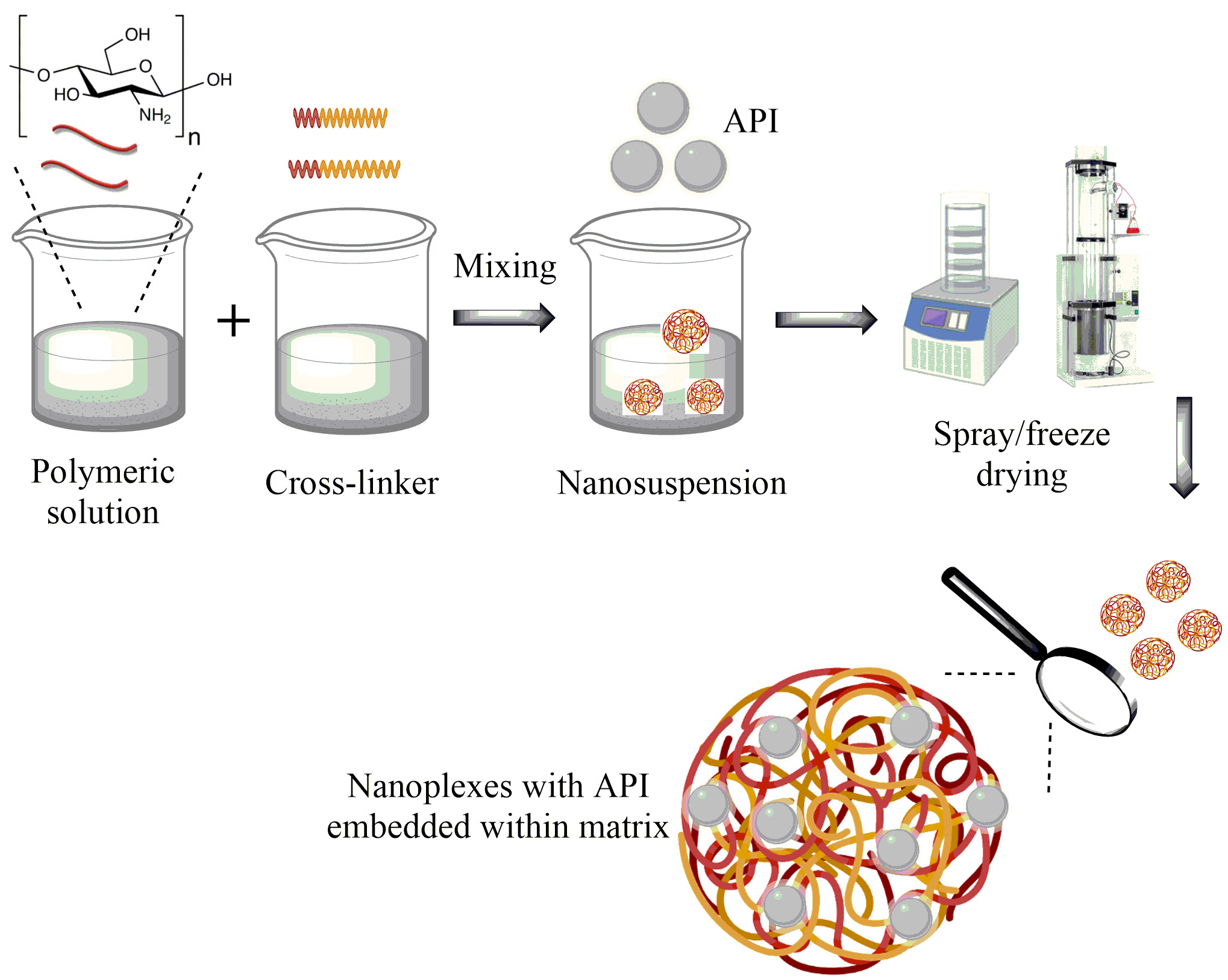
Natural polymers are extensively studied for their biocompatibility, however, their surface chemistry and charge can be exploited to obtain nanoplexes. Nanoplexes are the nano-dimensional cross-linked complexes obtained by the electrostatic interaction between the different polymers or; polymer and cross-linker [1]. Chitosan is the most widely used biopolymer known for its biodegradation. Nanoplexes can be easily synthesized by utilizing chitosan by the simplest ionic gelation method. Moreover, the nanoplexes can be loaded with an API during or after the cross-linking. In both cases, the API is embedded into the matrices of the nanoplexes allowing controlled release over time, following degradation of the polymer [2]. Nanoplexes are the latest drug delivery cargoes and can be designed as an ideal carrier for a variety of hydrophilic or hydrophobic drugs.
Natural polymers are extensively studied for their biocompatibility, however, their surface chemistry and charge can be exploited to obtain nanoplexes. Nanoplexes are the nano-dimensional cross-linked complexes obtained by the electrostatic interaction between the different polymers or; polymer and cross-linker [1]. Chitosan is the most widely used biopolymer known for its biodegradation. Nanoplexes can be easily synthesized by utilizing chitosan by the simplest ionic gelation method. Moreover, the nanoplexes can be loaded with an API during or after the cross-linking. In both cases, the API is embedded into the matrices of the nanoplexes allowing controlled release over time, following degradation of the polymer [2]. Nanoplexes are the latest drug delivery cargoes and can be designed as an ideal carrier for a variety of hydrophilic or hydrophobic drugs.
