SZTE Nanotechnological Research Group
Principal investigator: Dr. Rita Ambrus habil PhD
Particle engineering for formulation of dry powder inhalation systems applicable in lung diseases
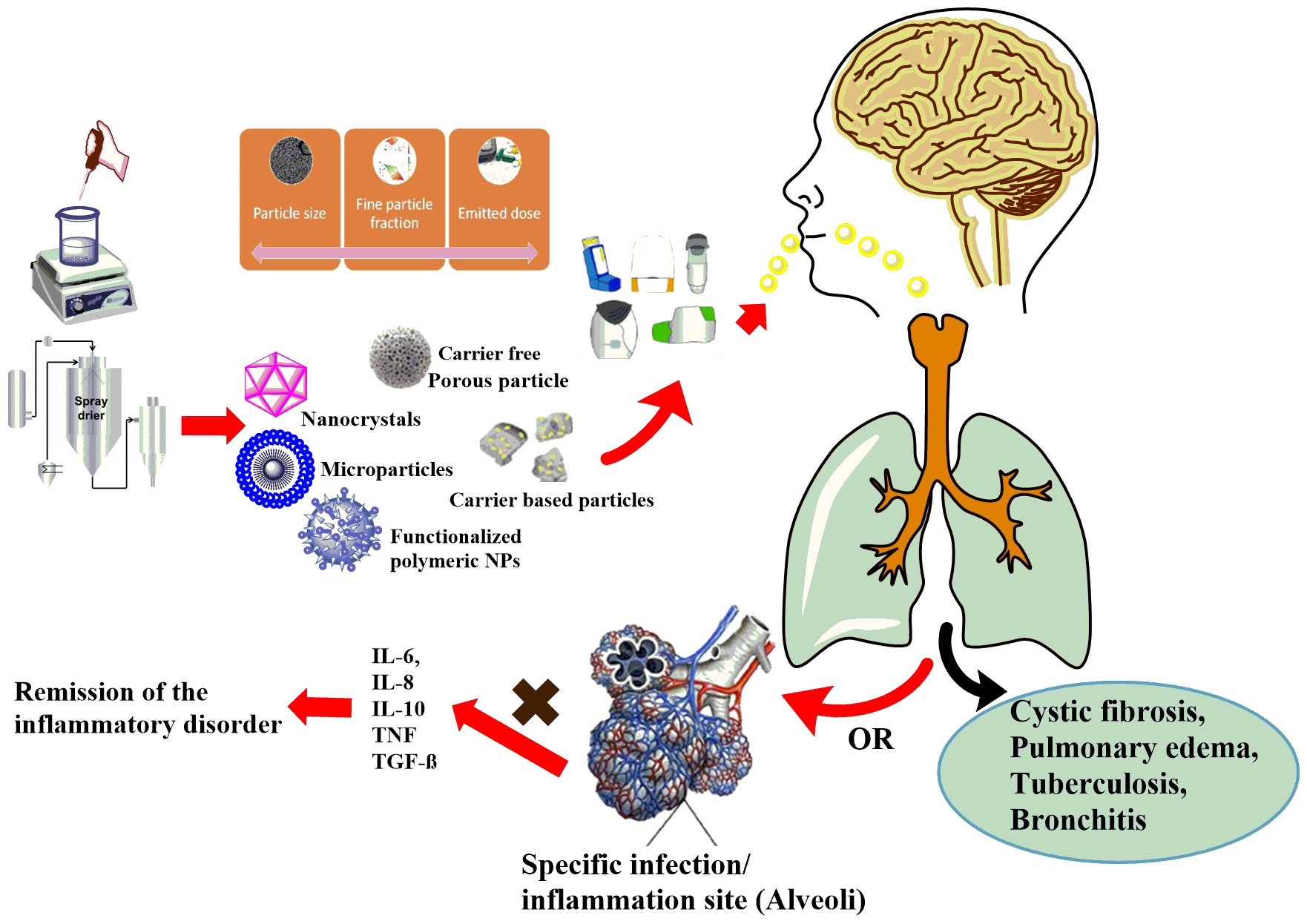
Pulmonary drug delivery (PDD) has several benefits over oral medications. Thus, it is becoming an alternative choice to treat local and systemic diseases. PDDs can be divided into three main categories (pressured metered-dose inhalers, nebulizers, dry powder inhalers /DPIs/). Recently, the development of DPI products observed due to many advantages. The international literature separates traditional, carrier-based and carrier-free DPI systems. Our research team is interested in developing both directions, as there are many opportunities to develop both.
Most of the DPIs available on the market are made with carrier-based formulation, which involves applying the active ingredient particles to the surface of a large carrier particle by forming an interactive physical mixture. The use of carriers is an advantage in the case of active ingredients that have a strong cohesive property, the flow properties of the composition are improved, applying the small doses of the active substance could be easier by dilution with the carrier, and the taste of the carrier confirms successful inhalation by the patient. There are still great opportunities in this line due to the surface modification possibilities of the carrier particles.
In the case of carrier-free DPI systems, the application of large carriers can be avoided with the use of special excipients and technologies (e.g. co-spray drying). These systems yield around 50–60% Fine Particle Fraction results due to the apparent high cohesive properties between the active ingredient's particles. These formulations involve the usage of different amino acids (e.g. L-leucine) and polymers (e.g. polyvinyl alcohol or polyvinylpyrrolidone). However, the main problem with the microparticles is that they stick to each other because of their high surface free energy. Overcoming this cohesive nature included the incorporation of large amounts of lipophilic adjuncts (such as the mixture of cholesterol and phospholipids e.g. sodium stearate). In addition to the various excipients used, formulation of nanosuspension and than nano spray-drying could be a new option for the preparation of carrier-free DPIs. Durring our work NSAID and antibiotic agents are used for the treatment of CF and COPD.
Dry powder inhalers (DPI) comprised of biodegradable polymeric nanocarriers with adequate aerodynamic profile, drug release and biocompatibility is a major challenge for pulmonary drug delivery.
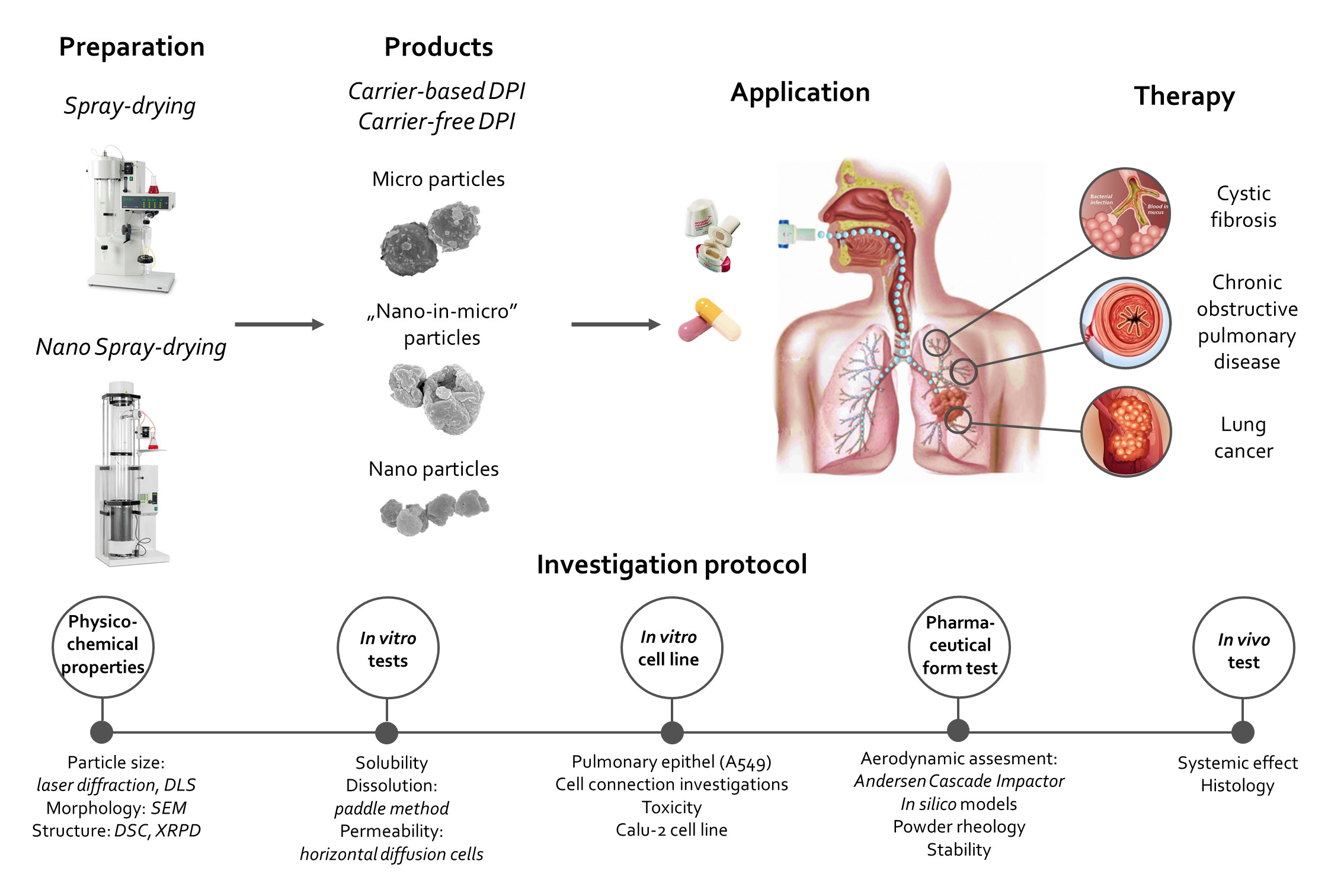
The aim of this research is to fabricate the targeted drug delivery nano vehicle for the active targeting of the inflamed pulmonary tissues. Different polymers and their derivatives are to be assessed to establish their potential in the targeted drug delivery. The rationale of the active targeted delivery is the sustained release of drug to the affected cells while minimizing the drug associated toxicity to the healthy epithelium. This targeted nanotherapeutics might be able to target the disease at the molecular level with regression and remission. This may lead to the inhibition of cascade of cellular events involved in the stimulation and aggravation of inflammation. The developed nano system is to be characterized for stability, cell viability, ex-vivo and in-vivo deposition in the inflamed tissues. Nanotechnology based DPI can emerge as a promising approach in the inflammatory and infectious disorders to circumvent the limitations of conventional formulations.

