SZTE Nanotechnological Research Group
Principal investigator: Dr. Rita Ambrus habil PhD
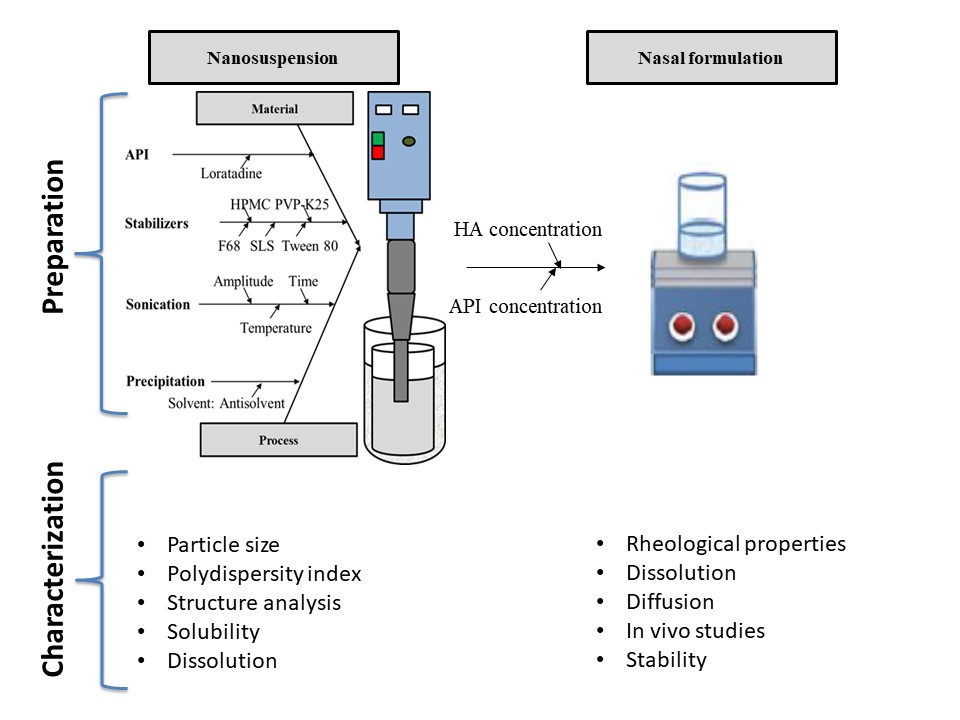
Nanosuspension-based nasal delivery of poorly water-soluble loratadine
During our work, the critical material such as drug amount, type and concentration of stabilizer, and solvent: antisolvent ratio. Besides, process parameters such as sonication time, power, and temperature were studied and optimized according to the previously determined quality of attributes, namely the particle size, polydispersity index, solubility, and dissolution. Further, the nasal formulations were prepared, and the concentration of the mucoadhesive agent (sodium hyaluronate) and the concentration of nanosized drug in the nasal formulation were studied. The formulations were characterized by referring to mucoadhesive properties, dissolution in simulated nasal fluid, diffusion, and in vivo pharmacokinetic parameters.
Loratadine nanosuspensions were successfully prepared using Poloxamer 188 as a stabilizer. The average particle size and polydispersity index were in the ranges of 264-312 nm and 0.16-0.18, respectively. The nanosuspension based formulation showed higher mucoadhesive properties, enhanced diffusion and improved bioavailability compared to the unprocessed loratadine.
References
[1] Alshweiat, A., Ambrus, R., Katona, G., Csoka, Ii., 2019. QbD based control strategy of loratadine nanosuspensions and dry nanoparticles stabilized by Soluplus®. Farmacia 67, 729–735. https://doi.org/10.31925/farmacia.2019.4.23
[2] Alshweiat, A., Katona, G., Csóka, I., Ambrus, R., 2018. Design and characterization of loratadine nanosuspension prepared by ultrasonic-assisted precipitation. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2018.06.010