SZTE Nanotechnological Research Group
Principal investigator: Dr. Rita Ambrus habil PhD
Application of co-milling procedures in wet and dry media for particle size decreasing
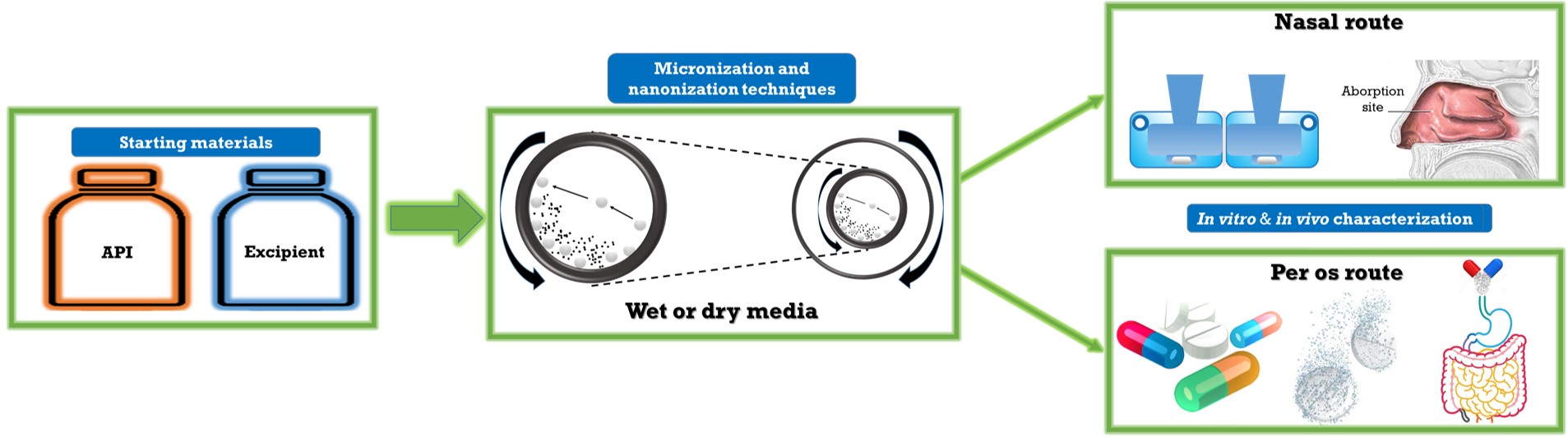
Milling is a commonly applied technique to produce micro- or nanosized drug crystals. The main effect of the process is the comminution of particles, which results in changes in physicochemical properties of the ground material. The combined wet milling process used also in this research work is a fusion of two basic milling techniques, in both cases the milling is performed in presence of grinding media (beads). However the disadvantages could be the presence of amorphous fraction.
One of the aims of this work was to study the wet milling process, where the planetary ball mill was combined with pearl milling technology and optimize the process parameters and predict the robustness of the process and design the amount of the additive (PVA) in order to produce Meloxicam containing nanosuspension without any pre-treating procedure. Furthermore, the optimized drug formulations were tested on the cell culture model of intestinal epithelium.
In other way, we produced dry powder formulation containing micro- or nanosized drugs (which have effect in central nervous system) with additives, as a further applicable intranasal formulations. Lamotrigine (LAM) is an antiepileptic agent with a relatively mild side effect spectrum, but only available in tablet form on market. Due to the dry milling technique, the particle size of LAM, their surface and also their structure changed that led to higher in vitro dissolution and permeability rate. The results of the in vivo tests showed that the axonal transport of the drug was assumable by both intranasal formulations because the drug was present in the brain within a really short time, but the LAM from the nanoLAMpowder liberated even faster.
The nasal administration of levodopa is a suitable possibility to treat the off-periods of Parkinson’s disease. As for stability problems of the aqueously dissolved drug, it has to be formulated in nasal powders. To reach it, the diameter of nasal powder particles is supposed to be in a 5-40 micrometer range. Taking these parameters into consideration, we are formulating nasal powders potentially appropriate for the treatment of off-periods by co-milling procedures using different excipients.
References
[1] Bartos, Csaba ; Ambrus, Rita ; Katona, Gábor ; Sovány, Tamás ; Gáspár, Róbert ; Márki, Árpád ; Ducza, Eszter ; Ivanov, Anita ; Tömösi, Ferenc ; Janáky, Tamás, Transformation of Meloxicam Containing Nanosuspension into Surfactant-Free Solid Compositions to Increase the Product Stability and Drug Bioavailability for Rapid Analgesia DRUG DESIGN DEVELOPMENT AND THERAPY 13 pp. 4007-4020. , 14 p. (2019)
[2] Bartos, C., Pallagi, E., Szabó-Révész, P., Ambrus, R., Katona, G., Kiss, T., Rahimi, M., Csóka, I. (2018). Formulation of levodopa containing dry powder for nasal delivery applying the quality-by-design approach. European Journal of Pharmaceutical Sciences, 123, 475-483.
[3] Kiss, T., Alapi, T., Varga, G., Bartos, C., Ambrus, R., Szabó-Révész, P., Katona, G. (2019). Interaction studies between levodopa and different excipients to develop coground binary mixtures for intranasal application. Journal of pharmaceutical sciences, 108(8), 2552-2560.
[4] Gieszinger, P ; Tomuta, I ; Casian, T ; Bartos, Cs ; Szabó-Révész, P ; Ambrus, R., Definition and validation of the Design Space for co-milled nasal powder containing nanosized lamotrigine. DRUG DEVELOPMENT AND INDUSTRIAL PHARMACY 44 : 10 pp. 1622-1630. , 9 p. (2018)

