SZTE Nanomedicine Research Group
Principal investigator: Prof. Dr. Ildikó Csóka
Projects
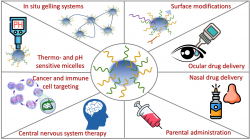
Polymeric micelles are versatile nano drug delivery systems built up by amphiphilic copolymers encapsulating a poorly water-soluble drug. With enhanced water solubility, the polymeric micelles can be used for (1) improving pharmacokinetic parameters, (2) increasing bioavailability and (3) deliver drugs to specific target areas. With lots of advantages e.g., biocompatibility, low toxicity, the nano size and high entrapment efficiency, these systems can be used for many drug delivery routes, such as nasal, ocular, parental, topical.
The current focus of our research deals with the auspicious nose-to-brain delivery pathway aiming to treat neurodegenerative disease-associated neuroinflammatory symptoms in the central nervous system and with the formulation of ocular in situ gelling systems to improve the drug release and absorption in the case of eye infections.
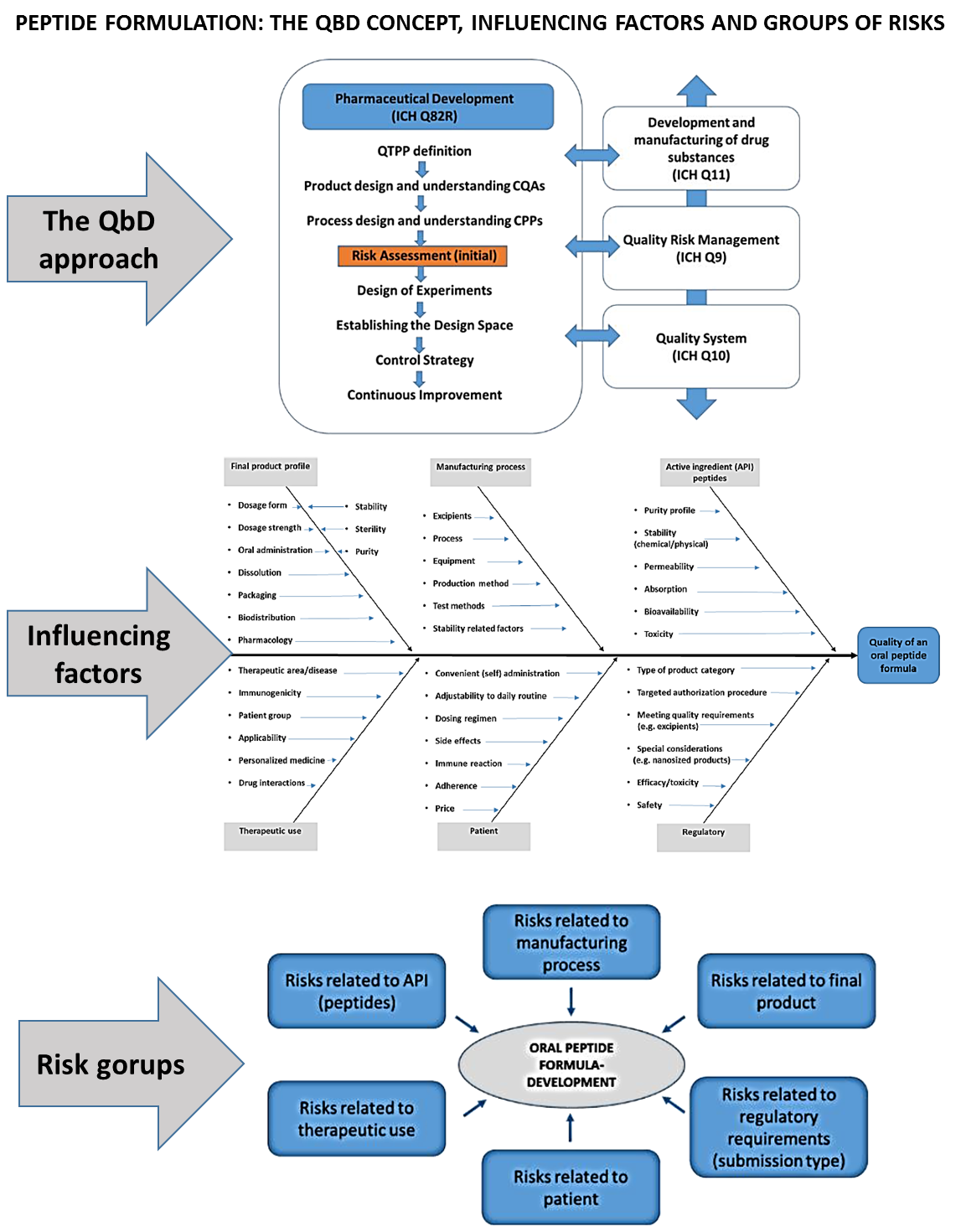
The Quality by Design (QbD) approach is a holistic, systematic, knowledge and risk-based methodology of the pharmaceutical development approach, which is proactive and focuses on the profound preliminary design.
QbD has several steps, which are described in the relevant guidelines of the International Council of Harmonisation (ICH), namely in the ICH Q8R2, Q9, Q10 papers.
The implementation of QbD in the manufacturing of medicinal products is often called the “GMP of the 21st century”; regulatory authorities strongly force the pharmaceutical industry to apply this strategic planning approach. The adaptation and application of this methodology into the early research phases within the R&D activities hold many advantages1, as QbD based early development brings scientific results closer to the practical requirements and has a facilitating effect on the industrial scale-up and product transfer to the market.
As QbD strategy is a Risk Assessment (RA) centered approach, where the experiments in practice are based on the critical factors with most highly critical effect on the aimed target product, the application of it in R&D projects23 is especially useful by the development of such complex formulations which need interdisciplinary thinking, like biologicals and peptide manufacturing4. In this case the extended QbD concept can be applied1 especially focused on the preformulation design.
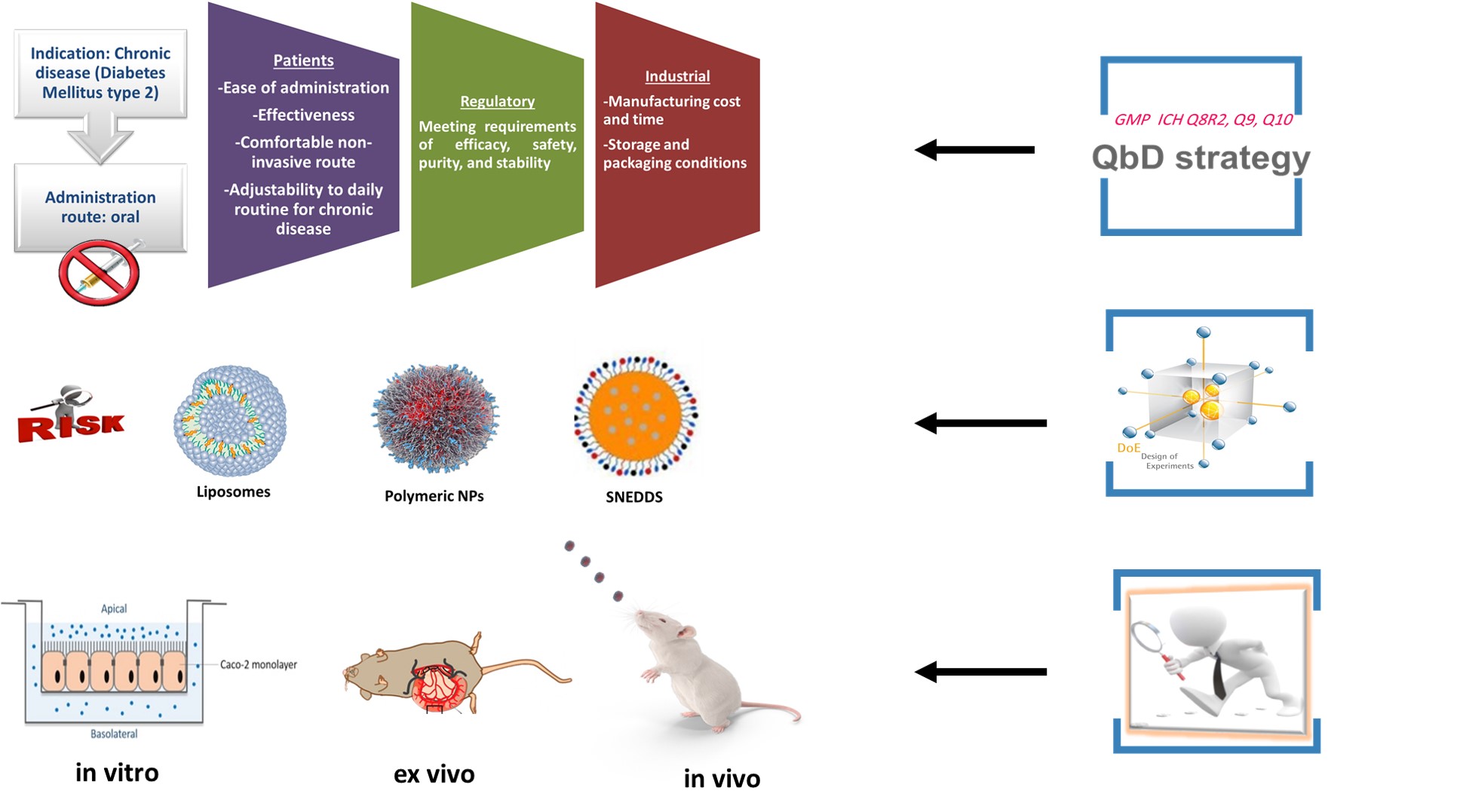
GLP-1 analogues, exenatide and liraglutide, are currently administered through subcutaneous injection which is an invasive route that would adversely affect patients’ adherence to the therapy. Thus, a great effort should be devoted to developing a patient-friendly delivery system, and the oral route is regarded as the most widely accepted means of administration as it could prove safe and effective. Additionally, the oral delivery stimulates the normal physiological pathway of the native GLP-1 peptide by targeting the liver, and subsequently minimizes potential side effects which would increase patients’ compliance to the treatment and thus increase the treatment efficacy. However, the oral delivery of peptide drugs constitutes a great challenge due to the enzymatic barrier in the gastrointestinal (GI) tract, and the absorption barrier presented by gastrointestinal epithelial cells and tight junctions between adjacent epithelial cells contribute to the poor diffusion and penetration of the peptides across the intestinal epithelium into the blood circulation, resulting in low oral bioavailability.
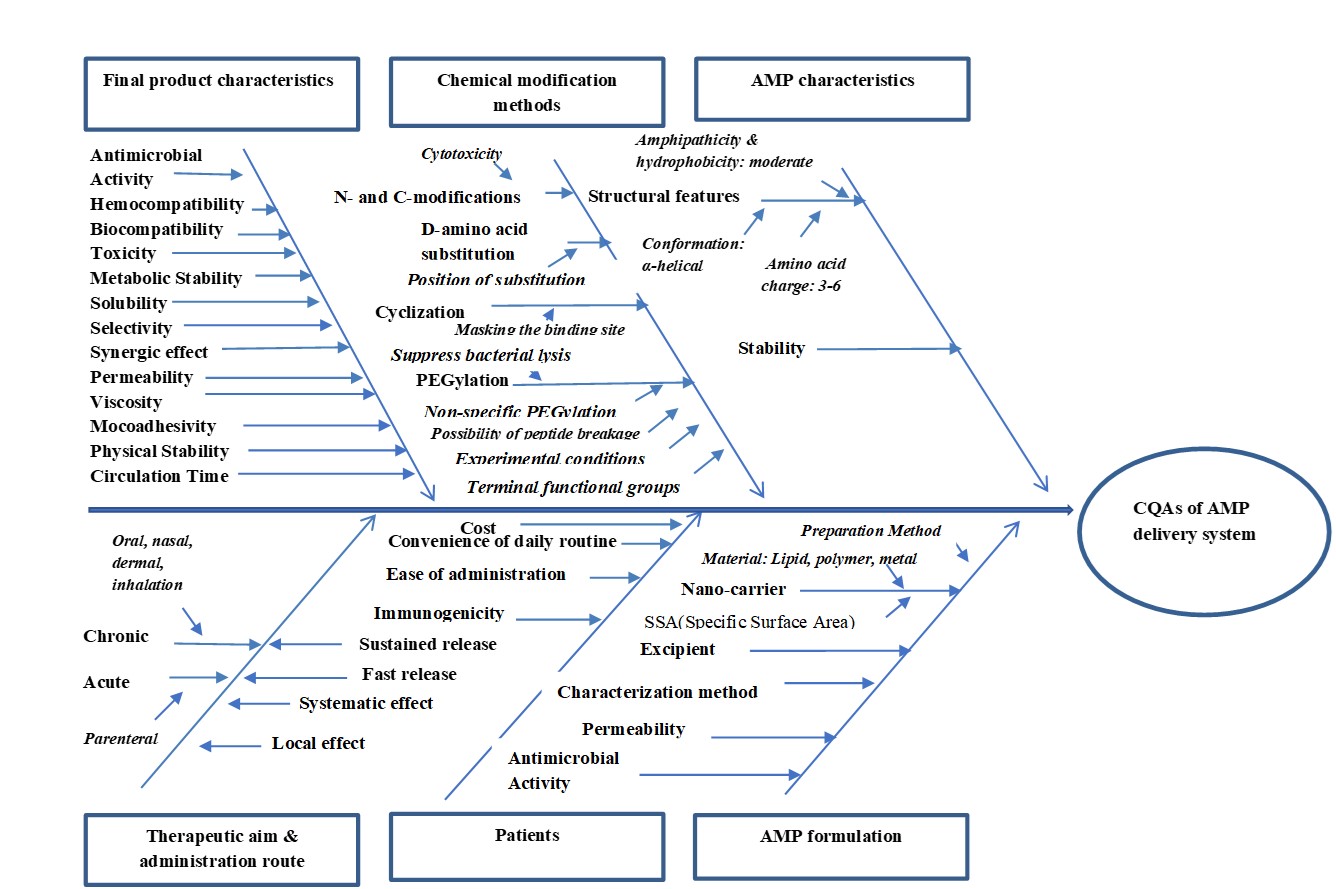
Antimicrobial peptides (AMPs) research activities are in the midpoint since the antimicrobial resistance is one of the main global threats according to the World Health Organization’s (WHO) report. AMPs can be categorized based on their source, target, structure, mechanism of action, therapeutic aim, modification and formulation methods. Despite the considerable advantages of AMPs in terms of safety aspects and low rate resistance development, several limitations such as low metabolic stability, low permeability across biological barriers, high costs and difficulty in reaching targeted sites at active concentrations are blocking the AMPs delivery.
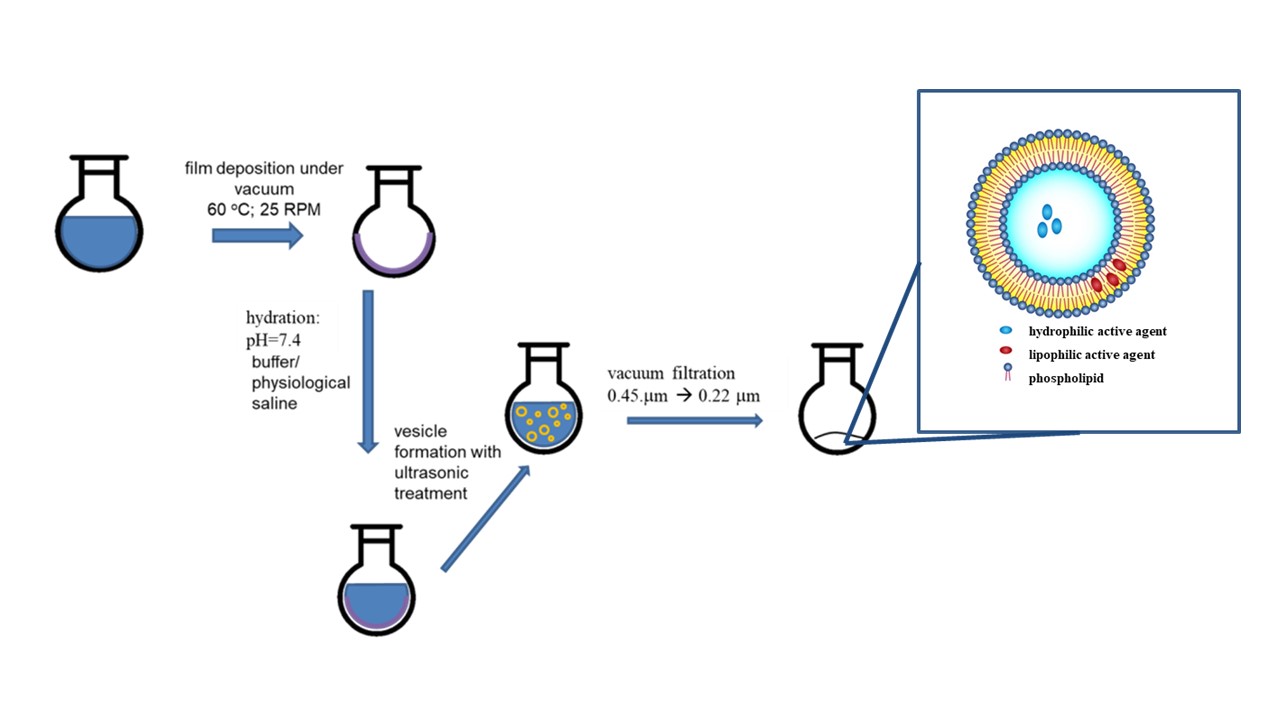
Liposomes are nanosize lipoidal vesicles that are under extensive investigation as drug carriers with improving the delivery of therapeutic agents. Liposomes can trap both hydrophobic and hydrophilic compounds. They have a lot of advantages. Because of their biocompatibility, biodegradability, low toxicity, and aptitude to trap both hydrophilic and lipophilic drugs and simplify site-specific drug delivery to tissues, liposomes have increased rate both as an investigational system and commercially as a drug-delivery system. Lately, liposomes became viable prospects as nanocarriers in applications, where an active agent cannot be directly applied due to either the need of being protected from decomposition or its poor solubility in solvent.
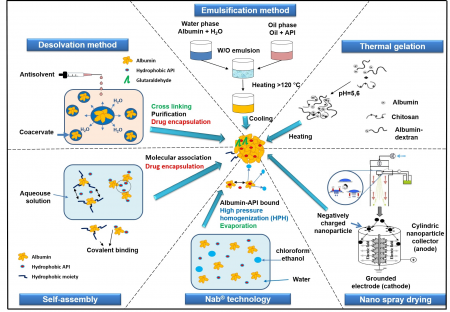
Albumin is playing an increasing role as nano drug carrier in the clinical therapy. Principally, three drug delivery technologies can be distinguished: coupling of low-molecular weight drugs to exogenous or endogenous albumin, conjugation with bioactive proteins and encapsulation of drugs into albumin nanoparticles. The accumulation of albumin in inflamed tissues and solid tumors forms the rationale for developing albumin-based drug delivery systems for targeted drug delivery.
The current focus of our research deals with the alternative route of administration of albumin nanoparticles which can be a promising tool for threating neurodegenerative diseases, whose present treatment is hindered because of the BBB protective mechanism.

