SZTE Nanomedicine Research Group
Principal investigator: Prof. Dr. Ildikó Csóka
Nanocarriers for non-invasive delivery of GLP-1 analogues - liraglutide and exenatide
GLP-1 analogues, exenatide and liraglutide, are currently administered through subcutaneous injection which is an invasive route that would adversely affect patients’ adherence to the therapy [1]. Thus, a great effort should be devoted to developing a patient-friendly delivery system, and the oral route is regarded as the most widely accepted means of administration as it could prove safe and effective. Additionally, the oral delivery stimulates the normal physiological pathway of the native GLP-1 peptide by targeting the liver, and subsequently minimizes potential side effects which would increase patients’ compliance to the treatment and thus increase the treatment efficacy [2]. However, the oral delivery of peptide drugs constitutes a great challenge due to the enzymatic barrier in the gastrointestinal (GI) tract, and the absorption barrier presented by gastrointestinal epithelial cells and tight junctions between adjacent epithelial cells contribute to the poor diffusion and penetration of the peptides across the intestinal epithelium into the blood circulation, resulting in low oral bioavailability [3].
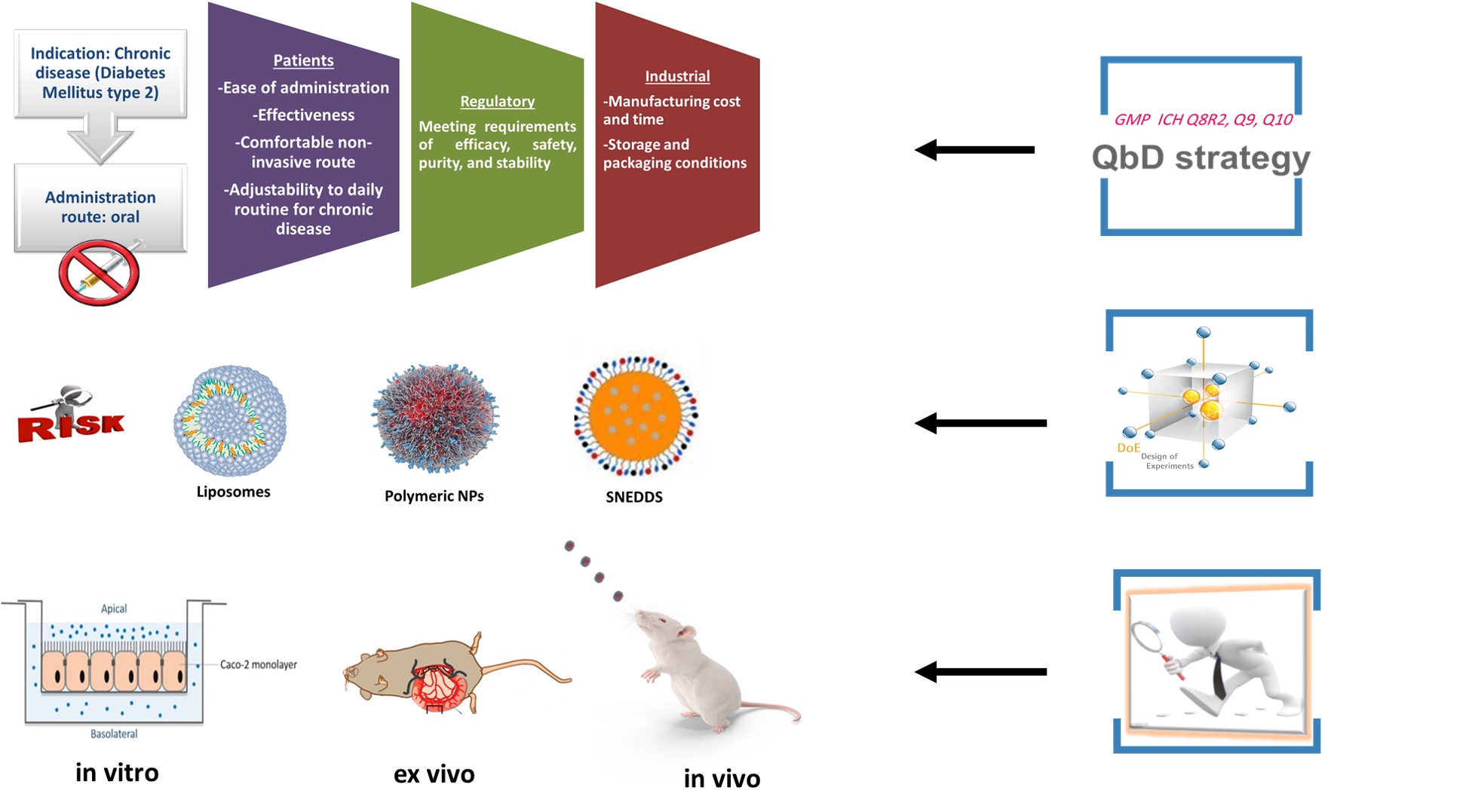
The main aim of this research project is to investigate the potential of different nanocarrier systems in overcoming the main barriers blocking the oral delivery of liraglutide and exenatide, namely the harsh environment in the GI tract and the absorption membrane barrier. Due of the complexity, biocompatibility and nanotoxicological concerns of nanopharmaceuticals in addition to the risks entailed with peptide drugs formulation development, it is critical to apply QbD approach to develop peptide-loaded into nanocarriers to classify product attributes and process parameters and ultimately develop a thorough understanding of the target product, process design, in order to save time and effort by directing the effort toward building the quality in each step of peptide carrier system development [4].
To achieve the mentioned aims, the main steps are as follows:
1. Literature review: A careful literature evaluation regarding the up-to-date applied techniques and strategies for oral delivery of antidiabetic peptides with more focus on understanding the characteristics of polymeric and lipid-based nanocarriers.
2. Pre-formulation: This could be done by introducing the elements of QbD, the GMP of the 21st century, and conducting an initial risk assessment study for developing oral GLP-1 analogues loaded nanocarriers.
3. Formulation development: Design and optimization of polymeric nanosystems and lipid-based nanosystems (self-emulsifying drug delivery systems, liposomes) that would comply with the predefined quality target product profile.
4. Investigations:
- Measurement of particle size, surface charge and encapsulation efficiency of the developed systems.
- Investigation of structural stability of liraglutide/exenatide loaded in nanocarrier (CD, FT-IR).
- Evaluation of in vitro release kineticfins and conducting enzymatic stability study.
- Conducting cytotoxicity and intestinal permeability study across the intestinal cell model.
- Conducting cytotoxicity and intestinal permeability study (rat intestinal mucosa).
- Pharmacokinetics study following in vivo study in rats
References:
1. Ruba Ismail and Ildikó Csóka. “Novel strategies in the oral delivery of antidiabetic peptide drugs – Insulin, GLP1 and its analogs,” Pharm Biopharm.,115,257-267,2017.
2. Ruba Ismail, Tamás Sovány, Attila Gácsi, Rita Ambrus, Gábor Katona, Norbert Imre, Ildikó Csóka. “Synthesis and statistical optimization of poly (lactic-co-glycolic acid) nanoparticles encapsulating GLP-1 analog designed for oral delivery,” Pharm. Res., 63,99,2019.
3. Ruba Ismail, Alexandra Bocsik, Gabor Katona, Ilona Grof, Maria Deli, Ildikó Csóka, Encapsulation in polymeric nanoparticles enhances the enzymatic stability and the permeability of GLP1 analog- liraglutide across a culture model of intestinal permeability. Pharmaceutics., 11,599, 2019.
4. Edina Pallagi, Ruba Ismail, Tamás Paál, Ildikó Csóka. “Initial Risk Assessment as part of the Quality by Design in peptide drug containing formulation development,” Eur J Pharm Sci.,122, 160–169,2018.

