Case 4.: Treatment of a young female advanced breast cancer patient with paclitaxel-bevacizumab therapy
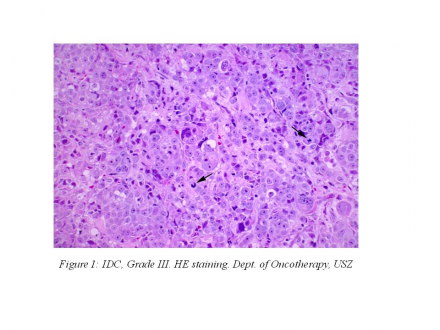
The 38-year-old female patient first appeared at our department on September 22, 2010, presenting with complaints that had lasted for one year: she had dyspnea, nausea, vomiting, back pain and she could not see with her left eye and could not open her left eye. Her ECOG status was 3 at that time. She brought along her histology result: invasive ductal carcinoma Grade III. (Figure 1)
ER:90%, PR:20%, HER2:+++ (with IHC). During physical examination we palpated a centrally ulcerated tumor that infiltrated the skin of the right breast (8x12 cm). In the outer, upper quadrant of the left breast a 5x7 cm tumor, in the outer, lower quadrant of the left breast a 3x4 cm tumor was palpable. There was a fixed lymph node conglomerate in the right axilla, above the lungs there was a dullness of 3 fingerbreadths on percussion, her liver was 2 fingerbreadths enlarged.
Staging examinations performed on September 23, 2010 revealed the following metastases:
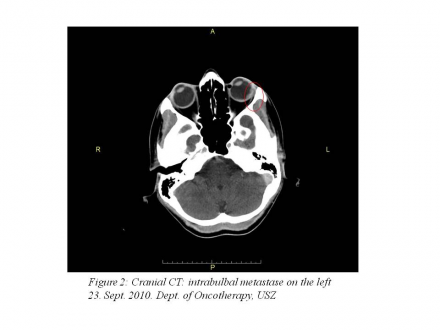
cranial CT: 10x3 mm soft tissue structure in the left bulb that corresponds to an intrabulbar metastasis (Figure 2).
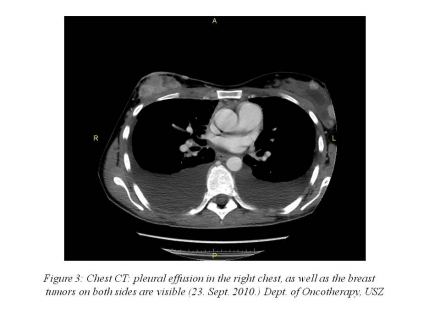
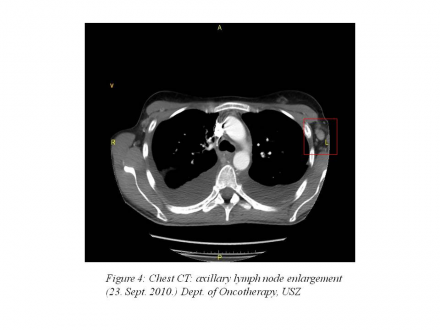
chest CT: 55 mm pleural effusion in the right chest, 45 mm pleural effusion in the left chest, complete atelectasis in the lower lobes of the lung, axillary and subcarinal lymph node enlargement (10-17 mm) (Figure 3 | Figure 4).
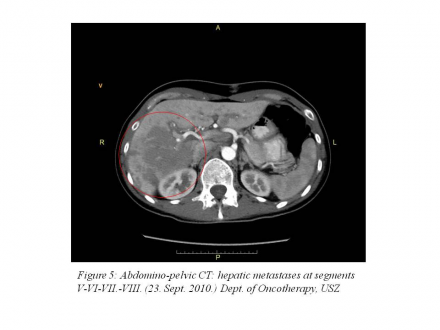
abdominopelvic CT: a a 130 mm metastasis in segments VIII, VII, VI and V of the liver and a 30 mm metastasis in segment I of the liver (Figure 5).
Bone scan performed on 24 September, 2010 showed multiple osseal metastases, so we immediately started the patient’s intravenous bisphosphonat treatment. Echocardiography revealed 74% EF and a 3-4 mm pericardial effusion. Her laboratory results showed elevated liver function values (GOT:80 U/l, GPT:50 U/l, AP:269 U/l).
We would have liked to treat the patient in an international trial, but the leaders of the trial asked for a repeated core biopsy that was performed on October 19, 2010. The histology result of the central laboratory was the following:IDC, Grade III. ER:90%, PR:20%, HER2 (FISH): negative.
Having the central laboratory result, we started the treatment of the patient, who was in an unchanged general state, on November 9, 2010 with palliative bevacizumab-paclitaxel treatment.We performed the treatment as follows: 80 mg/m2 paclitaxel iv weekly, 10 mg/kg bevacizumab iv every 2 weeks. (1 cycle = 2 weeks).
In cycle 5, the patient developed Grade II peripheral neuropathy, so we decreased the dose of paclitaxel by 20%. By this time the patient’s ECOG status was already 0. She could open her left eye and she could see with it again.
12 weeks after the first treatment we performed restaging examinations with the following results:
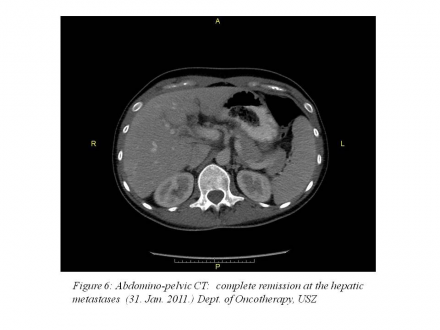
January 31, 2011: abdominopelvic CT: complete remission.(Figure 6)
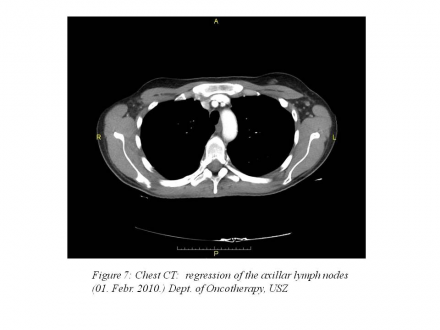
February 1, 2011: chest CT: pleural effusion 20 mm on the right side, 4 mm on the left side, 7-10 mm lymph nodes that are non-pathologic in size according to RECIST 1.1 criteria.(Figure 7)
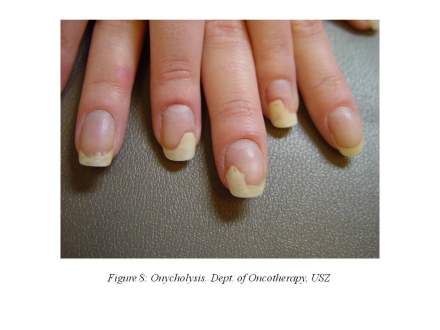
In cycle 8, the patient developed painful onycholysis (Figure 8),
so the dose of paclitaxel was further reduced by 20%.
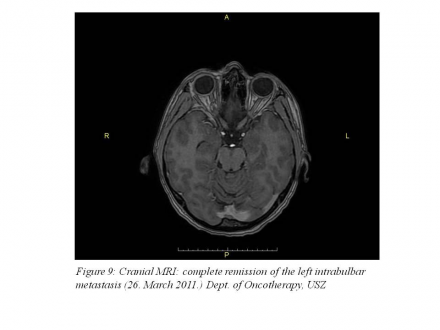
On March 26, 2011, cranial MRI examination was performed that showed the complete remission of the intrabulbar metastasis. (Figure 9)
After cycle 10, Grade III peripheral neuropathy developed and there was a repeated increase in the liver enzyme levels, so we suspended her palliative treatment. After performing the patient’s restaging examinations we received the following results:
Bone scan carried out on April 14, 2011 showed regression.
April 19, 2011: breast ultrasound: complete remission, the right breast had underwent automastectomy.
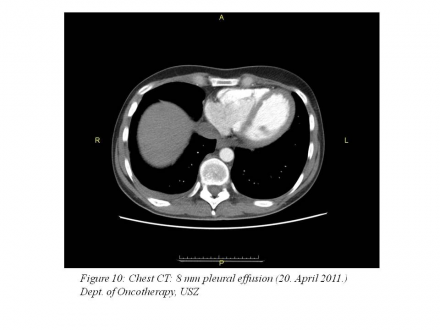
April 20-21, 2011: chest CT: 8 mm pleural effusion, otherwise SD (stable disease) (Figure 10),
abdominal status: negative.
Besides the side effects described above, the patient developed Grade II neutropenia as a side effect of paclitaxel, so she received GCSF (Granulocyte Colony Stimulating Factor) for 3 days after each treatment. The patient also had gingivitis, balance disorder and nose bleeding.
Since April 27, 2011, she receives palliative hormone therapy with LHRH analogue and tamoxifen.
Edited by Dr. Ágnes Dobi