Case 3.: Molecularly targeted therapy of advanced male breast cancer with capecitabine-bevacizumab combination
The 54-year-old male patient palpated a pea-sized lump in the left breast in December 2004. A cytological sample had been taken.
The cytological description contained C2, gynecomasty and epithelial hyperplasia and suggested the surgical removal of the lesion.
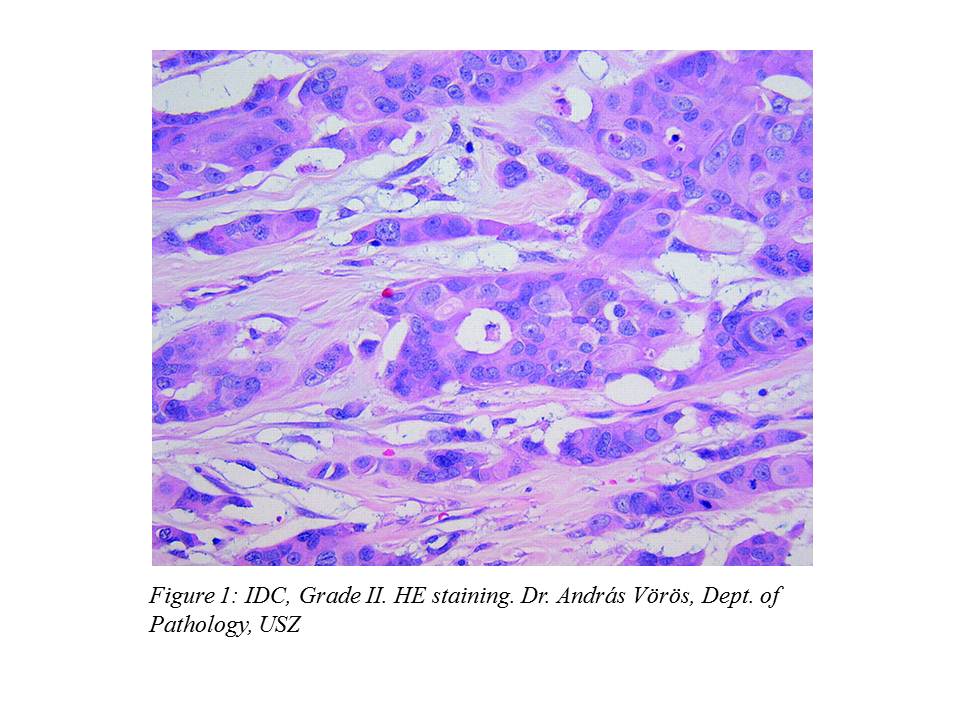
At the patient's explicit request, the operation was performed on March 4, 2005, where the intraoperative histology verified IDC. For this reason, according to the professional protocol, surgeons decided to carry out a left mastectomy and a left ABD (axillary block dissection) during the operation. The histological description of the surgical preparation was the following: IDC, Grade II. (Figure 1.), pT1 (13 mm), pN0 (0/14), ER:70%, PR:40%, cerb-B2: negative.
Following that, we offered postoperative radiotherapy to the patient at our outpatient department, which he had refused and we started adjuvant hormone therapy (tamoxifen), which he discontinued after 3 months. The patient did not participate in the obligatory oncologic outpatient care for years.
On December 4, 2008 the patient appeared at our outpatient department with a chest CT result that revealed multiple pulmonary (20-30 mm) metastases, mediastinal and hilar lymphadenomegaly (17-25 mm). The patient had no subjective complaints. Abdominopelvic CT (December 16, 2008) and bone scan, performed as staging examinations, were both negative. In December 2008 we started the patient’s palliative hormone therapy (tamoxifen). After an initial regression, chest CT on November 18, 2009 showed further pulmonary progression, so we suspended hormone therapy.
We offered participation in an international trial to the patient, which he accepted, so according to the study protocol repeated chest CT, abdominopelvic CT and bone scan were performed on December 8, 2009. Chest CT showed multiple lung metastases (the largest 29 mm), hilar and mediastinal lymphadenomegaly (21-35 mm) (Figure 2., Figure 3.).
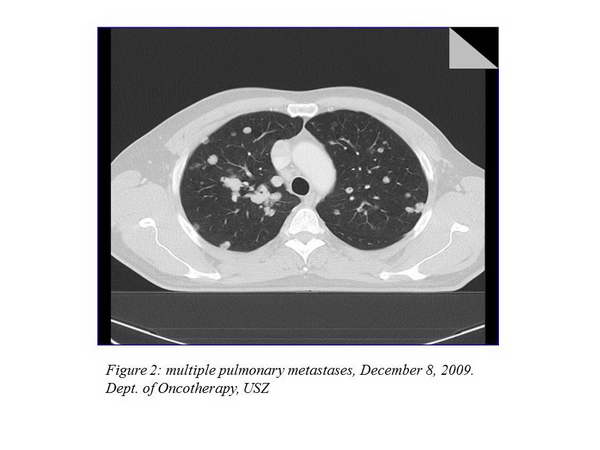
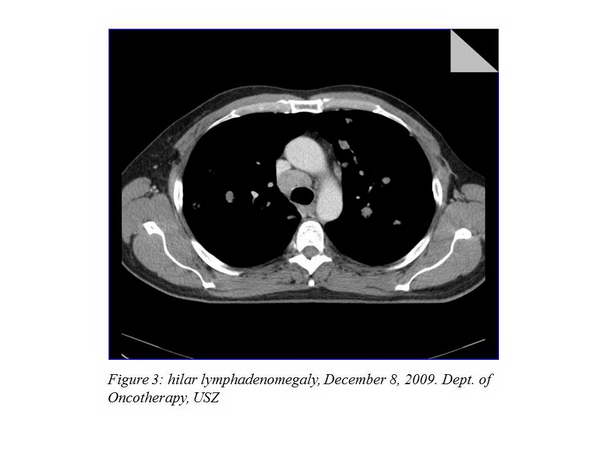
The other imaging examinations revealed no dissemination. As a complaint, the patient developed dyspnea, his ECOG status was 1. We started his palliative treatment on December 15, 2009 with bevacizumab-capecitabine treatment within the clinical trial.
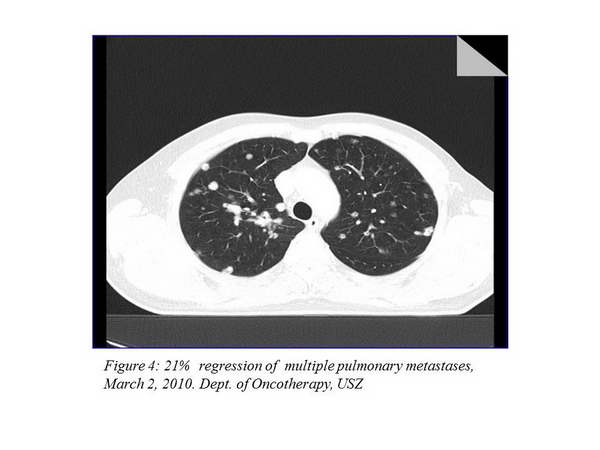
We carry out control imaging examinations every 12 weeks. The first re-staging examination took place on March 2, 2010, and it described a 21% regression in the thoracic status (Figure 4., Figure 5.).
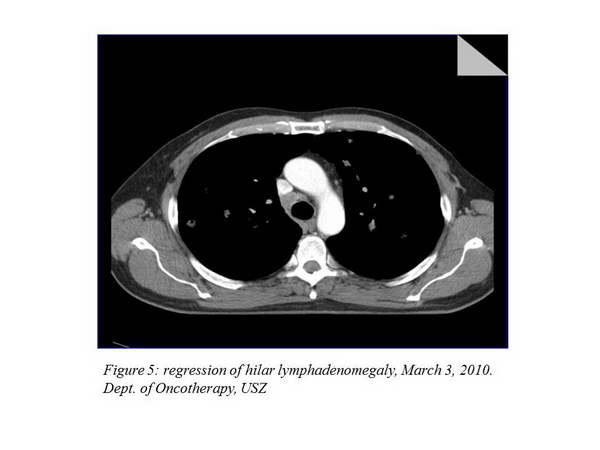
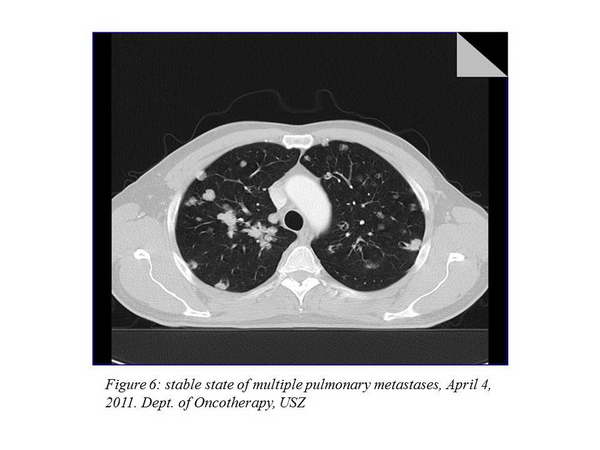
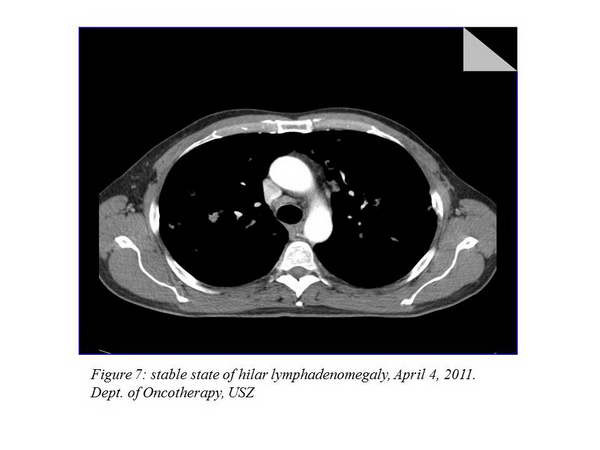
The next imaging examination 3 months later showed a further 7.8% improvement, the chest CT performed after that indicated a stable disease (there was no change evaluable in percentage). The other imaging examinations still did not to show any metastases at other sites. The chest status of April 4, 2011 can be seen on the figure below (Figure 6., Figure 7.).
The patient is presently still participating in the clinical trial, he had received 26 cycles of treatment. He receives treatments every 3 weeks as follows: bevacizumab 15 mg/kg iv, during 60 minutes. He takes capecitabine on days 1-14, in a dose of 2x2000 mg, then there is 1 week pause in taking the tablets. Since the beginning of the treatment, there was no cycle postponement or dose reduction. As a side effect, mild (Grade I) symptoms were developed: as a side effect of bevacizumab – hypertension and nose bleeding, as a side effect of capecitabin – hand-foot syndrome (HFS) (Figure 8.).
The patient is otherwise symptomless, his ECOG status is 0, he performs physical work in a car wash and leads a normal life.
This case demonstrates that the treatment is very effective (still “disease stabilisation” in September 2012), well tolerable, accompanied by only a few side effects, and a stable disease can be sustained in the long run.
Edited by Dr. Ágnes Dobi









